Bochum, 21. April 2021:
Vor 2 Tagen wurde im DGE-Blog über Publikationen zur Frage eines „Repurposing“ von Metformin bei COVID-19-Erkrankungen berichtet (siehe 1). Es wurden bisher schon weit über 200 bereits zugelassene oder sich für andere Indikationen in Entwicklung befindliche Substanzen auf etwaige Wirksamkeit bei COVID-19 getestet, jedoch bei über 95% wieder beendet, da ohne Effekt. Im Folgenden sei es gestattet, ebenso wie im Beitrag über Metformin (1), die gesammelten Daten in englischer Sprache zu präsentieren.
—
Therapeutic approaches in COVID-19
Mortality of hospitalized COVID-Patients is dropping:
– Other technical approach than in the beginning of the pandemia: No early ventilation.
– Heparin against thrombosis (present in 30-40% of the hospitalized patients)
– Dexamethasone now standard medication in severly ill (ventilated) patients
(at variance to ventilated flu patients)
Early onset of Antiviral therapy, already at the first symptoms when the viral load is high. Not much effect later, when inflammation dominates.
Remdesemir: better effect when given early (?),
Molnupiravir, developed against flu, also tested for COVID-19 in phase III
—
Repurposing of licensed drugs and drugs under development
Antiviral: Remdesevir (?, see above),
5 drugs against flu, other virustatics and still other drugs in phase II
Cardiovacular: Anticoagulants, hypotensive drugs
Depressant immunomodulators: Blockers for Interleukin, TNF-alpha, Brutontyrosinkinase, Januskinase*); from human microbioma, HIV drugs etc.
Pulmonary diseases: New drugs in Phase III and II, antibodies from cell cultures, other candidates
Endocrinology & Diabetes: Corticoids: Dexamethasone in severe cases (see above), Budesonide spray (in asthma and COPD) protective (?). Metformin, Raloxifen, Vitamin D.
*) Baricitinib, Tocilizumab, Sarilumab: used since years in Rheumatoid Arthritis
—
Drugs against COVID-19 in development (*emergency license in USA)
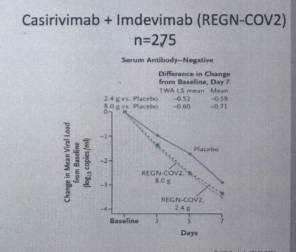
REGN-COV2, Regeneron and Roche: 2 monoclonal antibodies (MAB)
Casirivimab and Imdevimab, preventing cell penetration (given to Donald Trump).
See Fig.1
AZD7442, AstraZeneca: 2 long-acting MAB, blocking virus replication
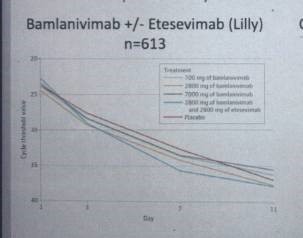
Bamlanivimab* Lilly: 1 MAB, binds to spike protein and should inhibit penetration. The emergency license for monotherapy was withdrawn by FDA in April 2021 for monotherapy because of virus resistence. In combination with Etesevimab, another MAB from Lilly, however, it is still effective. See Fig. 2.
TY027, Tychan: 1 MAB binds to virus and hinders its spreading
Ifenprodil (Algernon): Depresses immune T-cells and diminishes exacerbating immune reactions (cytokine storm). Tested also in Romania
VIR-7831, Vir Biotechnology and GlaxoSmithKline: 1 MAB, binds to virus hindering its replication
Molnupiravir (MSD): Developed against flu (see above) , active also in SARS.
Inhibits polymerases necessary for virus replication (similar to remdesivir)
Vilobelimab, InflaRx: New principle, developed in Jena, Germany.
Stops inflammation by inhibiting C5a protein components
Aviptadil, Relief Therapeutics. Artificially produced peptides (vasoactive intestinal polypeptides) acting similar to hormones, found in lung cells expanding the bronchial system. Stops also virus replication in the lung
Opaganib, RedHill Biopharma: Licensed as Yeliva® for bile duct carcinoma.
Inhibits SK2 enzymes which promote not only growth of carcinoma cells but also inflammation
Hydroxychloroquine, Zentiva: Licensed in the EU for Rheumatoid Arthritis and both prophylaxis and therapy of Malaria. In COVID-19 ineffective and side effects.
Stopped by WHO, FDA emergency license* withdrawn
Fig.1: Casirivimab + Imdevimab (REGN-CO2) (Gottlieb et al., JAMA 2021)
Fig.2: Bamlanivimab + / – Etesevimab (Lilly). Gottlieb et al., JAMA 2021)
Kommentar
Ein Durchbruch ist, wie auch bei anderen Viruserkrankungen, bisher nicht gelungen, trotz intensivster Forschungs- und Entwicklungsarbeit vieler Firmen und Institute. Erfreulicherweise stammt auch eine der vielen neuen Substanzen, das Vilobelimab aus Jena. Sonst ist Deutschland, einst die „Apotheke der Welt“ nicht vertreten. Zu viele bürokratische Vorschriften würden in unserem Land eine zügige Entwicklung von neuen Medikamenten behindern, meint man in dem deutschen Zentrum der Cochrane Foundation in Tübingen. Dafür sind wir bei der COVID-19 – Impfung mit der Vaccine Comirnaty von Biontech aus Mainz weltweit wohl Spitzenreiter. Hoffentlich folgt auch bald der Impfstoff der Tübinger Firma Curevac.
Helmut Schatz
Literatur
(1) Helmut Schatz: Metformin bei COVID-19 – aktuelle Studienlage.
DGE-Blogbeitrag vom 19. April 2021
Neueste Kommentare